Abstract
Background: Axi-cel is a US FDA-approved autologous anti-CD19 chimeric antigen receptor (CAR) T cell therapy for treatment of adult patients (pts) with relapsed or refractory large B cell lymphoma after ≥ 2 prior lines of therapy. In ZUMA-1, the pivotal study of pts with refractory large B cell lymphoma, the objective response rate (ORR) was 82%, including a 58% complete response (CR) rate (Neepalu and Locke, et al. N Engl J Med. 2017). Grade ≥ 3 cytokine release syndrome (CRS) and neurologic events were observed in 12% and 31% of pts, respectively, and were generally reversible. Checkpoint proteins, such as PD-1 and PD-L1, have been shown to be expressed on both CAR T cells and in the tumor microenvironment and subsequently upregulated after CAR T cell infusion (Vranic, et al. PLoS One. 2017; Cherkassky, et al. J Clin Invest. 2016; Galon, et al. ASCO 2017. #3025). This suggests that axi-cel activity could be augmented by incorporating PD-L1 blockade. This end of Phase 1 analysis of ZUMA-6 examines the safety and preliminary efficacy of axi-cel in combination with the anti-PD-L1 antibody atezolizumab (atezo) in pts with refractory diffuse large B cell lymphoma (DLBCL; NCT02926833).
Methods: Eligible pts (≥ 18 years) with refractory DLBCL, defined as stable or progressive disease to last line of therapy or relapse within 12 months after autologous stem cell transplant, must have recieved prior CD20-targeting and anthracycline-containing regimen and had ECOG ≤ 1 and adequate bone marrow and organ function. Pts received low-dose conditioning with fludarabine 30 mg/m2/day and cyclophosphamide 500 mg/m2/day × 3 days followed by axi-cel infusion at a target dose of 2 × 106 cells/kg. Atezo was administered at 1200 mg every 21 days for 4 doses starting on Day 21, 14, and 1 post-axi-cel infusion for Cohorts 1, 2, and 3, respectively. This report describes Phase 1 results from all 3 cohorts. Incidence of dose-limiting toxicities (DLTs) was the primary endpoint. Secondary endpoints included the frequency of adverse events (AEs), disease response, pharmacokinetics, and biomarkers.
Results: As of January 19, 2018, 12 pts have received axi-cel and at least 1 dose of atezo (3 in Cohort 1; 3 in Cohort 2, 6 in Cohort 3). Median age was 55 years (range, 30 - 66). Most pts (9/12, 75%) had received ≥ 3 prior therapies, and 4 pts (33%) had an International Prognostic Index score of 3 or 4. The median follow-up from axi-cel infusion was 4.4 months (range, 0.8 - 12.6), with 50% of pts having ≥ 6 months of follow-up. Eight pts (67%) have received all 4 doses of atezo, and 11/12 pts have received all scheduled doses of atezo. One pt in Cohort 3 experienced a DLT of Grade 4 thrombocytopenia and neutropenia lasting longer than 30 days. All pts experienced at least 1 AE (92% Grade ≥ 3), with no apparent exacerbation or recurrence of axi-cel-related toxicity following atezo infusion. Only 1 Grade ≥ 3 AE was attributed solely to atezo. Overall, the most common grade ≥ 3 AEs were anemia (9/12, 75%), encephalopathy (5/12, 42%), and neutropenia (5/12, 42%). Grade ≥ 3 CRS and neurologic events occurred in 3 (25%) and 6 (50%) pts, respectively. The ORR in evaluable pts was 9/10 (90%), with 6 pts (60%) in CR and 3 (30%) in partial response (PR); 2/6 pts (33%) had converted to CR at month 6 and month 9 after initially achieving a PR.
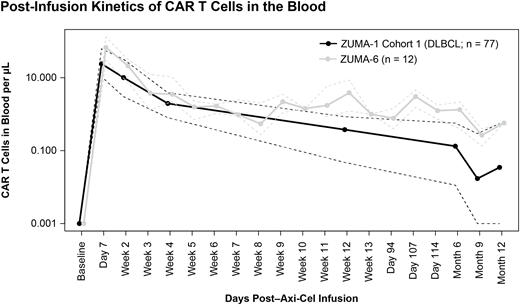
CAR T cell expansion as measured by area under the curve in the first 28 days (AUC0-28) was over 2-fold higher in ZUMA-6 than the median observed in pts with DLBCL in ZUMA-1 (ZUMA-6: median, 823 cells/µL × days, range, 99 - 2301; ZUMA-1: median, 357 cells/µL × days, range, 5 - 11,507; Figure). Median CAR T cell levels remained higher than ZUMA-1 beyond 28 days. However, initial peak CAR T cell levels were similar (ZUMA-6: median, 68 cells/µL, range, 9 - 274; ZUMA-1: median, 32 cells/µL, range, 1 - 1513). Interferon-γ (IFNγ) levels peaked within the first week after axi-cel infusion and reached a median of 730.5 pg/mL (range, 212 - 1876). The median peak IFNγ level in pts from ZUMA-6 was 1.5-fold higher than that from pts enrolled in Cohort 1 of ZUMA-1 (493.8 pg/mL, range, 32.4 - 1876).
Conclusions: PD-L1 blockade with atezo following axi-cel infusion has a manageable safety profile, with a low incidence of DLTs and no clinically significant evidence of increased incidence of AEs. Encouraging efficacy results support the opening of Phase 2 of ZUMA-6 in which 22 pts will be treated according to the Cohort 3 schedule. Pharmacokinetic data suggest the potential for enhanced CAR T cell expansion.
Locke:Kite Pharma: Other: Scientific Advisor; Novartis Pharmaceuticals: Other: Scientific Advisor; Cellular BioMedicine Group Inc.: Consultancy. Miklos:Kite - Gilead: Consultancy, Research Funding; Adaptive Biotechnologies: Consultancy, Research Funding; Pharmacyclics - Abbot: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Genentech: Research Funding; Janssen: Consultancy, Research Funding. Herrera:Merck, Inc.: Consultancy, Research Funding; Immune Design: Research Funding; Pharmacyclics: Consultancy, Research Funding; KiTE Pharma: Consultancy, Research Funding; Seattle Genetics: Research Funding; Gilead Sciences: Research Funding; AstraZeneca: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding. Westin:Apotex: Membership on an entity's Board of Directors or advisory committees; Celgen: Membership on an entity's Board of Directors or advisory committees; Novartis Pharmaceuticals Corporation: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees. Lee:Kite Pharma, Caladrius Biosciences: Employment; Kite Pharma, Caladrius Biosciences: Equity Ownership; Kite Pharma: Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Rossi:KITE: Employment. Zheng:Kite Pharma: Employment. Avanzi:Kite Pharma: Employment. Roberts:KITE: Employment. Sun:Kite, a Gilead Company: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal